A Nonsurgical Approach to Treating Prostate Cancer
Prostate cancer (PC) is a major health problem in the Western world being the second most common male malignancy in the European Union. The age-standardized incidence of PC varies more than one hundredfold between areas with the highest incidence (e.g., the United States) and the lowest incidence (e.g., Japan) in the world. In certain parts of the world, in particular the Western countries, the incidence of this disease has increased rapidly over the past 20 years. There are several environmental and genetic factors that partially explain these variations, although the incidence probably depends most of all on the extent to which small latent tumors are detected. Being that the clinical significance of small tumors is uncertain, the value of early diagnosis and early aggressive treatment is controversial.[note]see reference 2[/note]
More than half of patients with diagnosed PC do not have metastatic disease. This may be the result of better and earlier screening processes.[note]see references 3, 4[/note] Common treatment of PC with local manifestations includes radical prostatectomy and lymphadenectomy. More than 50 percent of all patients who are diagnosed with a tumor stage of T1-2 are free of lymph node involvement and metastatic disease. If surgery is not possible, radiation therapy is recommended. In advanced cases, androgen blockades are used such as gonadotropin-releasing hormone and/or orchidectomy. Treatments, such as prostatectomy, orchidectomy, and radiation therapy, yield a high risk of incontinence and impotence, making these treatments less than ideal.[note]see references 1, 3[/note]
This article provides an overview of local hyperthermia, discusses key studies, and then details the authors’ study of 184 patients with PC who were treated with transurethral hyperthermia (TUH) and complete androgen blockade (CAB).
1. Overview of Local Hyperthermia
Local or regional hyperthermia is the use of heat applied directly to an area of the body; for cancer treatment the heat is applied to a tumor. This kind of hyperthermia has been used alone or in combination with such therapies as chemotherapy and radiation for treating a wide variety of cancers in addition to PC, with excellent results. These cancers include melanomas, esophageal or stomach cancers, pancreatic cancers, sarcomas, bone tumors, cervical cancers, head and neck cancers, and rectal cancers. Hyperthermia causes direct cytotoxicity in the tumor, enhances its radiosensitivity, and improves the effectiveness of many chemotherapeutic agents in drug-resistant cells.[note]see reference 4[/note] Hyperthermia kills cancer cells selectively (because cancer is more sensitive to heat than healthy cells are). This is achieved via:
- Tissue acidosis
- Destruction of tumor capillaries
- Damage to tumor membranes and proteins
- Failure of cellular-repair mechanisms
- Changes in DNA and RNA synthesis
- Increase of free radicals
- Failure of antioxidant systems
- Induction of heat shock proteins (HSPs), which help to “unshield” a tumor, making it more susceptible to destruction by immune factors[note]Observation from Dr. Douwes’ clinical experience.[/note]
It is important to note that only microwave hyperthermia has been approved for legal use in the United States by the Food and Drug Administration and that it must be given with radiation therapy.
Immunotherapy of mice with preexisting cancers, using HSP preparations derived from autologous cancer, has retarded progression of the primary cancers, reduced metastatic loads, and prolonged the animals’ lifespans.[note]see references 6, 7[/note] HSP may have immunologic effects because the heat-inducible HSP70 expressed on the cell surface of certain tumor cells acts as a positive recognition signal for natural-killer cells. Therefore, in addition to causing direct cell cytotoxicity, hyperthermia has the additional potential for use in immunotherapy in clinical applications.
Several experimental data are available demonstrating the efficacy of combining interstitial hyperthermia and conformal radiation therapy.
Substantial clinical data exist, demonstrating that combining radiation and hyperthermia to treat early stage and advanced tumors of different types was more efficacious and produced less normal tissue toxicity compared to radiation therapy alone.
Phase I and II trials achieved impressive results in terms of objective response rate, local tumor control, and relapse-free survival for hyperthermia used alone or in conjunction with radiation therapy. Phase II trials have also produced excellent results using hyperthermia with chemotherapy and phase HI trials are currently underway. Most of these trials are being conducted in Europe.[note]see reference 5[/note] Most studies have used microwave hyperthermia to heat tumors to a temperature of 42.5°C-45°C. Other forms of hyperthermia include electric field, magnetic field, and radiofrequency hyperthermia. Higher temperatures appear to produce the best results.
2. Key Hyperthermia Studies
It has been suggested that a temperature of 43°C or higher is the optimum temperature to induce the most tumor destruction via apoptosis during hyperthermia. Apoptosis is an important mode of death in heated cells. No significant apoptosis was induced in PC-3 cancer cells when they were heated at 42°C for 240 minutes. Apoptosis is not an important mechanism of death in irradiated PC cells although radiation therapy is used to treat all stages of PC. Clinical and radiobiologic evidence indicate that PC cells are relatively resistant to radiation.[note]see reference 9[/note]
Several experimental data are available demonstrating the efficacy of combining interstitial hyperthermia and conformal radiation therapy.
2.1 Thermoradiotherapy Study
Researchers from the German Research Society conducted a phase II trial to determine the efficacy of thermoradiotherapy, using interstitial cobalt-palladium thermoseeds for treating patients who had localized PC. Forty-one (41) patients with localized PC were enrolled in the study between July 1997 and April 2000. Interstitial hyperthermia induced in a magnetic field was applied in six sessions, once per week. Conformal three-dimensional radiation therapy was given simultaneously in daily fractions. Intraprostatic temperatures ranged between 42°C and 46°C.
No major side-effects were observed during the hyperthermia sessions. The median levels of prostate-specific antigen (PSA) decreased from 11.25 ng/mL to 0.88 ng/mL 3 months after treatment and to 0.38 ng/mL 12 months after treatment with a median follow-up of 10 months. The mean prostate volume decreased from 32.6 mL to 26 mL after 3 months of treatment and to 18.5 mL after 12 months. A significant PSA decrease was observed in the subjects. The researchers concluded that interstitial hyperthermia is a feasible, well-tolerated procedure for PC therapy.[note]see reference 10[/note]
2.2 Local Microwave Hyperthermia Study
For another study, short- and long-term results of treating patients with stage III and IV PC were compared in 147 patients who did not have distant metastases. Thirty-eight (38) patients were exposed to radiation and 109 patients underwent radiation treatment and local microwave hyperthermia. The response of the primary tumor in each subject was evaluated via palpation, ultrasonic investigation, and computed axial tomography (CAT) scan. Adjuvant local hyperthermia enhanced antitumor activity of the treatment, raising the rate of a complete response from 69 percent with radiotherapy alone to 94 percent with thermoradiotherapy (hyperthermia plus radiation). Five (5)-year survival in patients with PC at stages III and IV increased from 48 percent to 65 percent.[note]see reference 11[/note]
2.3 Transrectal Ultrasound Applicator Study
A study using a transrectal ultrasound applicator for delivering prostate hyperthermia was combined with radiation therapy to treat 26 patients whose median age was 69. Nine (9) patients had well-differentiated adenocarcinoma, 11 patients had moderately differentiated adenocarcinoma, and 6 patients had poorly differentiated adenocarcinoma. All of the patients in this study had American Urologic Society Stage C2-D1 adenocarcinoma. The median pretreatment PSA level was 29 ng/mL. Hyperthermia was administered concurrently with radiation therapy at temperatures of 42.5°C for 30 minutes, using a transrectal ultrasound applicator with 3 thermometry probes, administered as either a single treatment (for 9 patients) or as two treatments (for 17 patients).
The median time to PSA nadir was 15 months, with a median PSA value of 1.0 ng/mL. The median and 5-year overall survival was 88 months and 73 percent respectively, and the median and 5-year biochemical no evidence of disease (bNED) survival was 36 months and 35 percent respectively. Multivariate analysis revealed only the pretreatment PSA level (P = 0.03) and the PSA nadir (P<0.01) to be significant predictors of bNED survival. The duration of hyperthermia therapy showed a trend toward significance for overall survival (P = 0.06). This phase I/II protocol, evaluating the combination of prostate hyperthermia and external beam radiation therapy for treating patients with locally advanced PC suggests that prostate hyperthermia is feasible with no apparent significant increased toxicity.[note]see reference 12[/note]
2.4 Radiofrequency Interstitial Tumor Ablation Hyperthermia Study
For yet another study, radiofrequency interstitial tumor ablation (RITA) hyperthermia was used as a sole method of treatment for inpatients with localized PC.
In 10 patients whose mean age was 70.4, a total of 21 marker lesions were biopsied under general, spinal, or local anesthesia. Radiofrequency energy was delivered transperineally under transrectal ultrasound guidance. Radical prostatectomy was performed on all patients 1-7 days after RITA.
The findings of intraoperative transrectal ultrasound and histologic examination of the specimens were correlated. Lesions 2 x 2 x 2 cm were targeted. Postoperatively, patients were catheterized for an average of 1.8 days.
Average lesion diameters, defined by coagulative necrosis at histologic examination, were 2.20 ± 0.23 x 2.10 ± 0.31 x 2.38 ± 0.14 cm (average volume 5.86 ± 1.63 cm ). Each lesion was well-defined and did not extend beyond the prostatic capsule. No complications (e.g., rectal-wall injury) were noted. RITA-biopsied lesions were safe, feasible, technically simple, and resulted in lesions that were very predictable in size and location. On histologic examination, well-defined areas of coagulative necrosis were documented. No damage to the periprostatic tissue was noted.
The procedure can be performed with spinal or local anesthesia only.[note]see reference 13[/note] This study suggests that RITA can destroy a tumor internally, forms scar tissue, will not harm nontumor tissue, and may reduce the need for radical prostatectomy.
2.5 Transrectal Hyperthermia
Transrectal hyperthermia (TRHT) was administered during a 15-month period to 15 patients with progressive adenocarcinoma of the prostate (i.e., PC). There were 5 patients with stage T4 disease and 11 with stage T3 disease, including 6 patients with skeletal metastases. Nine (9) of the patients had severe and 6 had moderately severe signs and symptoms of PC. Treatment was given six times, with a temperature of 43.5°C for 30 minutes. Cell-mediated immunity tests were performed before TRHT and at 2, 4, and 6 months post-therapy.
The results of these tests were compared to those of 15 patients with benign prostatic hyperplasia (BPH) who were treated with the same TRHT and with 30 untreated normal volunteers. TRHT was well-tolerated, with mild acute toxicity noted in 3 patients. Of the 15 patients who were treated, 2 (13 percent) had scintigraphic evidence of regression of bone metastases. Five patients survived more than 5 years since treatment and 3 patients had no evidence of PC. A decrease of marked or moderate degree of PC signs and symptoms was noted in 8 patients (53 percent).
The results of cell-mediated immunity tests were of interest. The 15 patients with PC, prior to TRHT, had a lower OKT4-OKT8 ratio and lower concavalin-A T-cell suppressor activity compared to the 15 patients with BPH and the 30 healthy volunteers who had normal immune parameters. This demonstrated significantly suppressed immune function. Following TRHT, there was a significant increase in monitored immune parameters noted in the 15 patients with PC (P<0.01). This immune stimulation peaked at 2 months and gradually decreased to near pretreatment levels at 6 months.[note]see reference 14[/note]
This study demonstrates augmentation of immune function as a direct consequence of hyperthermia in patients with PC with lowered immune function.
2.6 Thermoimmunotherapy
An early study combined immunotherapy with hyperthermia (thermoimmunotherapy) in patients with late-stage prostate or breast cancer. Propionibacterium granulosum KP-45 was used as the immunomodulator. The results, after 1-2 years of observation, indicated that the number of responders was higher in the groups treated with thermoimmunotherapy than in those who were treated with hyperthermia only. The researchers suggested that thermoimmunotherapy offers a real alternative for treating advanced neoplasms and opens a new field for experimental and clinical investigation.[note]see reference 15[/note]
Researchers suggested that thermoimmunotherapy offers a real alternative for treating advanced neoplasms and opens a new field for experimental and clinical investigation.
Table 1: Staging of PC in 184 Patients Enrolled in Study
| Diagnosis/age range | Stage | Number of patients |
|---|---|---|
| Prostate cancer, histologically verified, patients’ age range 50-87 |
TI.NO, MO | 18 |
| T2a, NO, MO | 16 | |
| T2b, NO, MO | 14 | |
| T3, Nx, MO-x | 136 |
Despite these excellent results, thermoimmunotherapy appears not to have been followed up in subsequent studies.
3. Materials and Methods for the TUH Study
Patients had refused prior surgery or radiation because of their anxiety about the procedures and fear of complications.
To test the efficacy of radiofrequency TUH, 184 patients, at the Klinik St. Georg, Bad Aibling, Germany, ages 50-82, with histologically verified PC were staged (see Table 1). Patients with either early stage (T1) or later-stage (T3) PC participated in this study. These patients had refused prior surgery or radiation because of their anxiety about the procedures and fear of complications, such as incontinence, impotence, metastasis, castration, retrograde ejaculation, anesthesia-related problems, infection, and bleeding. Psychologic reasons also played a role in these patients’ refusal to receive surgery or radiation treatment. Informed consent was recorded for all patients.
Treatment was performed with the Thermex II (Direx Medical Systems, Ltd. Peta Tikva, Israel) using a transurethral antenna. (This antenna is used to apply heat directly to the area around the urethra and provides continuous measurement of the highest temperature, allowing safe and effective distribution of heat throughout the prostate.)
Patients received a total of 2-3 treatment sessions, with each session lasting 1 hour under local anesthesia. Prostate temperatures were between 47°C and 48°C for the duration of each session. Monitoring of the temperatures was performed via direct measurement at the thermal electrode.
The patients also received CAB for 6 months, using the combination of luteinizing hormone-releasing hormone antagonists, androgen antagonists, or 5-ocreductase inhibitors. The subjects were monitored regularly in an outpatient department. Checkups were performed at 6 weeks, 3 months, 6 months, and 12 months. Monitoring was performed regularly via clinical examinations, laboratory tests, and appropriate imaging techniques (CAT scans, magnetic resonance imagings, bone scans, and positron emission tomography scans).
Table 2: Objective Results as Demonstrated in Prostate Glands of 184 Patients After TUH with CAB
| Tumor stage | Number of patients | Gland smaller | Gland softer | No change in gland | Gland larger or harder | |
|---|---|---|---|---|---|---|
| TI.NO-I MO | 16 | 5 | 8 | 8 | 0 | |
| T2b, NO, MO | 32 | 15 | 21 | 11 | 0 | |
| TI-4, Nx, MO-x | 136 | 81 | 77 | 55 | 0 | |
| Total | 184 | 101 (55%) | 101 (55%) | 74 (40%) | 0 |
Subjective and objective micturition parameters improved significantly in more than 60 percent of the patients.
4. Results
Six (6) weeks after treatment with TUH and CAB, 55 percent (101 of 184) of the subjects had smaller and softer prostate glands, as noted via rectal examination, and 40 percent had no palpable change (see Table 2). Prostate volume was reduced from an average of 33 mL to 25 mL in early stage disease and from 56 to 33 mL in advanced cases (see Table 3), which was confirmed by transrectal sonography. In addition to reduction in size, the disappearance of typical signs of cancer and the appearance of scar tissue in place of the tumors were observed.
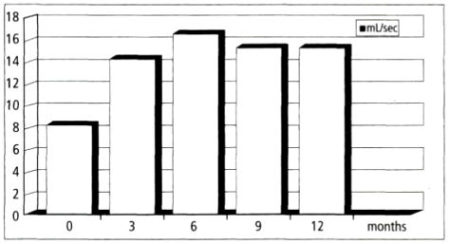
Ten patients were randomly biopsied and 8 of 10 (80 percent) were negative for PC. Subjective complaints, such as micturition, poor flow rates, voiding dysfunction, residual volume, incontinence, and nocturia were reduced, as shown in Figures 1 and 2.
The average flow rate increased from 8 mL to 14 mL at 3 months, rose to 17 mL at 6 months after treatment, and remained at 15 mL at months 9 and 12 (Figure 1).
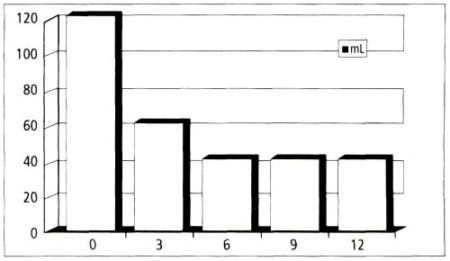
The average residual volume, which was measured by ultrasound, was reduced from 120 mL to 60 mL at 3 months and remained at 40 mL at 6 months, 9 months, and 12 months after treatment (Figure 2).
Voiding time was reduced an average of 70-42 seconds at 6 months. Although 5 patients had had total urinary retention, all patients improved to the degree that their catheters could be removed. The subjective and objective micturition parameters improved significantly in more than 60 percent of the patients.
One hundred percent of patients with early stage prostate cancer experienced complete remission during the twelve-month observation period.
Three (3) months after treatment, the total number of remissions observed was 91 of 184 (49.5 percent), the total number of partial responses was 28 of 184 (15.0 percent), no change or progressive disease was 29 of 184 (15.2 percent); and progressive disease was 36 of 184 (19.6 percent) (Table 4). Patients at the earliest stage of PC, with stages Tl, NO, MO, 16 of 16 (100 percent), had complete remissions. Patients with early stage disease, T2 a-b, NO, MO, 32 of 32 (100 percent), had complete remissions. Patients with late-state disease, 43 of 136 (31.6 percent), had complete remissions (Table 4). In patients at the later stages of PC, Tl-4, Nx, MO-x, 43 of 136 (31.6 percent) had complete responses, 28 of 136 (20.5 percent) had partial responses, 29 of 136 (21.3 percent) had no change and no progression of disease, and 36 of 136 (26.4 percent) had progressive disease.
Table 3: Prostate Volume After TUH and CAB in 184 Patients with PC
| Tumor stage | Number of patients | Volume (mL) before treatment | Average volume (mL) at 3 months | Volume (mL) after 6 months | Volume (mL) after 9 months |
|---|---|---|---|---|---|
| TI,NO-x, MO | 16 | 30 | 28 | 27 | 25 |
| T2a-b, NO-1, MO | 32 | 34 | 29 | 25 | 22 |
| TI-4, Nx, MO-x | 136 | 56 | 41 | 36 | 33 |
The total response of all groups was 91 of 184 (49.5 percent), complete response; 28 of 184 (15 percent), partial response; 29 of 184 (15.2 percent), no change (and no progressive disease); and 36 of 184 (19.6 percent), progressive disease. Remission was verified by imaging techniques and laboratory tests.
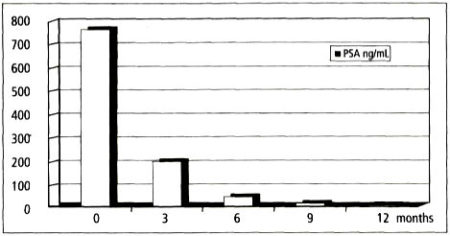
In those patients who experienced complete remission 91 of 184 (49.5 percent), the PSA value progressively dropped on average from 750 ng/mL to 200 ng/mL at 3 months, to 30 ng/mL at 6 months, and to <5 ng/mL at 9 and 12 months (Figure 3). As reported in earlier literature, [note]see reference 12[/note] hyperthermia is effective for lowering PSA levels to normal in patients who respond to treatment.
All patients who did not live near the clinic were instructed to seek follow-ups by their urologists and to have follow-up reports sent to the clinic. The clinic followed all local patients. While it is oftentimes difficult, if not impossible, to follow patients for 5 years, 38 of the 43 patients with late stage PC who had complete remissions were followed for 5 years with no serious symptoms after 5 years. Two (2) patients died of causes unrelated to PC. Five (5) patients had local recurrences and were treated with TUH and CAB with complete remission observed and confirmed via biopsy, laboratory tests, and appropriate imaging analysis.
Table 4. Response of 184 Patients with PC to TUH and CAB
| Tumor stage | Number of .patients | CR | PR | NC | PD |
|---|---|---|---|---|---|
| TI,NO-x, MO | 16 | 16 (100%) | N/A | N/A | N/A |
| T2a-b, NO, MO | 32 | 33 (100%) | N/A | N/A | N/A |
| TI-4, Nx. MO-x | 136 | 43 (31.6%) | 28 (20.5%) | 29 (21.3%) | 36 (26.4%) |
| Total | 184 | 91 (49.5%) | 28 (15%) | 29 (15.2%) | 36 (19.6) |
Apparently, this combined treatment does not make PC resistant to subsequent treatment unlike radiation and chemotherapy. Sixteen (16) of the 16 patients who had early stage PC were followed and 2 died of causes unrelated to PC. None of these patients had recurrences within this 5-year period. Urinary incontinence and impotence were not reported side-effects as a result of this treatment. While libido decreased in some patients during CAB therapy, libido returned to pretreatment levels (or better than pretreatment levels in some patients) after cessation of CAB therapy.
5. Discussion
TUH to the prostate combined with CAB produced excellent results for patients with early stage PC, with 100 percent experiencing complete remission during the 12-month observation period. For early stage PC, removal of the prostate gland and/or radiation plus androgen blockade is the current conventional treatment of choice. However, this treatment modality carries significant risk of producing incontinence and/or impotence. In addition, survival of these patients appears to be similar whether they are treated or untreated, casting some doubt upon enhancement of survival.
TUH is an effective, yet nonaggressive, treatment that improves micturition parameters and selectively kills PC cells without damaging surrounding tissue. Radiation therapy is not as selective and secondary cancers are not uncommon consequences of radiation therapy.
Surgery carries the greatest risk of producing incontinence and impotence relative to radiation therapy. TUH therapy carries none of these risks but rather reduces all PC symptoms.
In our study sample, all patients were able to have catheters removed, even patients with late-stage disease who did not respond to treatment. CAB appears to augment TUH therapy, given the overall response rate of 184 patients, most notably, the 100-percent remission in patients with early stage PC, a result that has not been reported in previous studies.
Early adjuvant hormonal therapy has been shown in retrospective studies to have a significant impact on time to progression and to cause specific survival in patients with seminal-vesicle invasion and limited lymph-node disease who undergo radical prostatectomy.[note]see reference 16[/note] Hormonal therapy plus TUH appears to be a viable alternative to radical prostatectomy for patients with PC. Furthermore, unlike surgery or radiation, TUH appears to have a direct impact on lymph-node invasion perhaps via production of HSP and immune activation.
Higher intratumor temperature and maintenance of intratumor temperature for a longer period of time can achieve greater results.
The authors feel strongly that the major benefit of TUH using radiofrequency is the ability of this therapy to raise prostate temperature to 47°C-49°C. It has been shown that optimal “killing” temperatures are at or above 43°C. Most of the studies reviewed did not exceed temperatures of 43°C.[note]see references 10, 25[/note] Also, during the 30-minute treatment sessions, the 43°C temperature was maintained for a short time—less than 5 minutes.[note]see references 10~15[/note]
Finally, most of the studies only performed one hyperthermia treatment for 30 minutes as the treatment protocol[note]see reference 10, 1[/note] with or without concurrent radiation treatment. None of the studies had intraprostate temperatures between 47°C and 49°C for 2-3 treatments lasting 1 hour per session,[note]see reference 10[/note] as described in this study. Time and temperature are both factors for optimizing hyperthermia treatment,[note]see reference 10[/note] the results of this TUH with CAB study.
Masunaga and colleagues studied the thermal clearance rate (TCR) of many different types of tumors, using local hyperthermia.[note]see reference 17[/note] Tumors were heated twice per week for 30-60 minutes per session up to a total of 2-11 sessions. Blood flow in the tumors was evaluated from the rate of TCR. Changes in TCR were closely related to average tumor-center temperature, changes in tumor number (as evaluated by CAT scan), and tumor response.
When smaller and more superficial tumors were treated by hyperthermia, combined with radiation and/or chemotherapy that consisted of many heating sessions, and during which a high average tumor-center temperature was achieved, a better tumor response was obtained. The better the tumor response was, the higher the local control rate became. The specific survival rates of patients who had good tumor responses were higher than those of patients who had poor tumor responses.[note]see reference 17[/note]
It is clear that higher intratumor temperature and maintenance of intratumor temperature for a longer period of time can achieve greater results. Unlike surgery and radiation, TUH also enhances immune function, induces apoptosis in PC cells only, and produces HSPs that assist in tumor recognition by unshielding tumors and making them more susceptible to immune-system attack.
TUH also significantly lowers PSA to normal levels in patient responders as reported previously.[note]see references 10, 12[/note] While 3 sessions of hyperthermia reduced signs and symptoms of PC in 53 percent of patients with advanced PC in these studies, no complete remissions were reported, although 2 of the 15 patients had bone metastases disappear.
This TUH with CAB study showed that 31.6 percent of patients with advanced PC had complete remissions, 20.5 percent has partial remissions, and 21.3 percent had no progression of disease.
6. Future Research
There are fewer side-effects associated with this treatment.
Because all patients at early stage PC in this study responded to this treatment protocol with 100-percent remission in this group, the authors agree that TUH with CAB is an excellent first-line therapy with far less risk than more aggressive therapy involving prostatectomy. While the results patients with later stage PC were still as good as or better than conventional therapy, there are also fewer side-effects associated with this treatment. In addition, unlike what occurs with adjuvant chemotherapy and radiation, patients do not appear to develop resistance to subsequent treatment if this becomes necessary.
However, it is important to find ways to increase the remission and response rate in patients with later-stage PC. Immunotherapy may be the missing link as reported by Szmigielski,[note]see reference 15[/note] and, in Germany, there are many products approved for augmenting immune function in patients with cancer. The addition of immunotherapy to this treatment protocol has yielded promising results at the Klinik St. Georg for patients with late stage PC. Immunotherapy is used as part of the clinic’s protocol for treating many types of early and late stage cancer, producing excellent results. Future research will be directed toward adding immunotherapy to the treatment protocol for patients with later stage PC, with the hope of achieving statistically significant results.
References
- Hamdy, F.C. Prognostic and predictive factors in prostate cancer. Cancer Treat Rev 27(3): 143-151, 2001.
- Sandblom, G., Varenhorst, E. Incidence rate and management of prostate carcinoma. Blom & Pharmacother 55(3): 135-143, 2001.
- Arrizabalaga Moreno, M., Garcia Gonzalez, J.I., Diez Rodriguez, J.M., Esteban Artiaga, R., Castro Pita, M., Navarro Sebastian, J., Mora Durban, M., Paniagua Andres, P. Epidemiologic indicators of adenocarcinoma of the prostate: Results on 436 patients [in Spanish]. Adas Urol Esp 21(9): 852-861, 1997.
- Soulie, M., Villers, A., Grosclaude, P., Menegoz, F., Schaffer, P., Mace-Lesech, J., Sauvage Machelard, M., Molinier, L., Grand, A. Cancer of the prostate in France: Results of the survey CCAFU-FRANCIM [in French]. Prog Urol 11(3): 478-85, 2001.
- Issels, R. Hyperthermia combined with chemotherapy – biological rationale, clinical application and treatment results. Onkologie 22: 374-381, 1999.
- Manjili, M.H., Wang, X.Y., Park, J., et al. Immunotherapy of cancer using heat shock proteins. Front Biosci 7: D43-D52, 2002.
- Wu, W., Liu, K., Gao, Y. Purification of HSP70 and its immunoprotective effect against mouse hepatoma. Zhonghua Zhong Liu Za Zhi 22(6): 96-90, 2000.
- Overgarrd, J. The current and potential role of hyperthermia in radiotherapy. Int J Radiat Oncol Biol Phys 16: 535-549, 1989.
- Li, W.X., Franklin, W.A. Radiation- and heat-induced apoptosis in PC-3 prostate cancer. Cells. Radiat Res 150(2): 190-194, 1998.
- Deger, S., Bohmer, D., Turk, I., et al. Thermoradiotherapy with interstitial thermoseeds in treatment of local prostatic carcinoma: Initial results of phase II study. Urologe A 40(3): 195-198, 2001.
- Tkachev, S.I., Bukharkin, B.V., Sholokhov, V.N., Klimakov, B.D. The comparative efficacy of radiation and thermoradiation treatments in prostatic cancer. Urol Nefrol (Mosk) 1: 25-28, 1996.
- Algan, 0., Fosmire, H., Hynynen, K., et al. External beam radiotherapy and hyperthermia in the treatment of patients with locally advanced prostate carcinoma. Cancer 89(2): 399-403, 2000.
- Djavan, B., Susani, M., Shariat, S., et al. Tranperineal radiofrequency interstitial tumor ablation (RITA) of the prostate. Tech Urol 4(2): 103-109,1998.
- Stawarz, B., Zielinski, H., Szmigielski, S., et al. Transrectal hyperthennia as palliative treatment for advanced adenocarcinoma of prostate and studies of cell-mediated immunity. Urology 41(6): 548-553, 1993.
- Szmigielski, S., Jeljaszewicz, J., Pulverer, G. Thermoimmunotherapy of advanced neoplasms: A concept and preliminary results. Biomecl Phannacother 41(3): 132-138, 1987.
- Zincke, H., Lau, W., Bergstralh, E., Blute, M.L. Role of early adjuvant hormonal therapy after radical prostatectomy for prostate caner. Urol 166(6): 2208-2215, 2001.
- Masunaga, S., Ono, K., Mitsumori, M., et al. Clinical usefulness of determining the rate of thermal clearance within heated tumors. Clin Oncol 26(6): 428-437, 1996.
Authors
Friedrich R. Douwes, M.D., is the founder and director of the Klinik St. Georg Integrative Cancer Treatment Center, Bad Aibling, Germany.
Shari Lieberman, Ph.D., C.N.S., F.A.C.N., is a research scientist and industry consultant in New York City.
Download
You can download this article: Download PDF



