Whole body hyperthermia (WBH) is the deliberate heating of the whole body to achieve an elevated core temperature of 41-42°C for a shorter time frame (average of 2 hours) or 39-40°C (average of 4 to 8 hours). There are clinical trials all over the world, including in the US.
These trials demonstrate that WBH along with chemotherapy can have remarkable effects in late-stage cancers with advanced solid tumors, advanced refractory or recurrent cancers, mesothelioma, advanced metastatic sarcoma, advanced metastatic cancers (GI, breast, head and neck, sarcoma, neuroendocrine), ovarian cancer, metastatic colorectal cancer, pediatric renal cell carcinoma, metastatic cervical cancer, metastatic sarcoma, metastatic melanoma, nodular lymphoma, chronic lymphocytic leukemia, and a host of other late-stage, chemo-resistant metastatic cancers.
Most studies use a lower therapeutic dose of chemotherapy, since WBH has a synergistic effect. It can lower the effective dose and overcome multidrug resistance. Also, several trials have shown that WBH has a synergistic effect with radiation and can lower the effective dose, making the radiation therapy less damaging. Unfortunately, WBH is only being studied in advanced, refractory, and multidrug-resistant patients. Yet the results have been remarkable. WBH is being used all over the world, including in Austria, Eastern Europe, Japan, China, and most notably Germany as part of an integrative cancer treatment.[note]see note 1[/note]
Using heat to treat disease dates back to ancient times

Application of fire to cure breast cancer is recorded in an ancient Egyptian papyrus. The therapeutic value of elevated body temperature by fever was also recognized by Hippocrates, who wrote: “What medicines do not heal, the lance will; what the lance does not heal, fire will.” Parmenides stated: “Give me a chance to create a fever and I will cure any disease.” Hot baths are considered therapeutic in Egypt, Greece, Rome, China, and India and also among many aboriginal tribes.
German physicians in the 19th century observed regression of sarcoma in patients who suffered prolonged, high fevers due to infectious disease. William Coley, a famous physician from New York City, used bacterial endotoxins, nowadays known as Coley’s toxins, to induce fever and cure many types of cancer. Elevated body temperature has been recognized as a cure for cancer and other diseases for centuries.[note]see note 1[/note]
Advantages of IR-A wavelengths
The majority of WBH systems being used employ infrared (IR) radiation to achieve systemic heating. A period of steady temperature increase is followed by a plateau phase wherein the target temperature is maintained for 30 minutes to several hours, followed by a cool-down phase. The patient is normally sedated to induce sleep during the procedure.
In German clinics, though, the device mainly used is the WBH-2000 unit and Heckel HT 3000. These are chambers that enclose all but the patient’s head. Special light-emitting diode radiators deliver computer-generated, water-filtered IR-A wavelengths that penetrate the skin to deliver heat to the capillary bed. These units are generally used because they have been shown to preferentially stimulate the immune system (Figure 1).[note]see note 1[/note]
In the US, oftentimes microwave radiation units are used instead. These have minimal impact on stimulating immune function and are dangerous: they need to be extremely focused and controlled because they can cause hot spots and burn damage, whereas infrared units will not. IR-A is a wavelength that specifically stimulates mitochondrial function; microwave radiation does not.
Therapeutic Effects of WBH
The effects of hyperthermia on natural killer (NK) cell activity have been examined in animal models. In a recent review article, WBH was shown to enhance NK cell cytotoxicity against tumor cells that is in part responsible for the improved clinical responses that are seen when hyperthermia is combined with other therapies.[note]see note 2[/note] Animal studies have shown that WBH increases chemotherapeutic agent uptake (for example, liposomally encapsulated doxorubicin) into tumors. It also may increase the number of perfused blood vessels over a prolonged period, making it useful for improved tumor targeting of cancer therapies.[note]see note 3[/note]
Moreover, WBH induces heat shock proteins (HSP) that can induce anticancer immune responses by targeting associated tumor antigens to the immune system. HSP do not only carry antigens but can also induce naturation of dendritic cells, resulting in a more efficient antigen presentation.
Hyperthermia has also been shown to have important stimulatory effects on several cellular and organismal endpoints related to the immune system.[note]see note 4[/note] Dendritic cells are antigen presenting cells that play a central role in the generation of effective antitumor immunity. Not only do they have the potential to recruit, select, and expand T cells that are specific for tumor cell antigens within lymphoid organs, but tumor-associated or vaccine-delivered dendritic cells may also help recruit T cells to the tumor site and help maintain their survival once they arrive.[note]see note 5[/note] Since dendritic cells have been shown to be thermally sensitive, using WBH in combination with immunotherapy strategies that are known to depend upon these cells such as vaccination may offer a superior benefit than using immunotherapy alone.[note]see note 5[/note]
Hyperthermia may also play a role in gene therapy.
Inadequate systemic delivery of functional DNA seriously limits current cancer gene therapy. In an animal model, WBH was shown to enhance the efficacy of suicide gene therapy through selectively increased tumor gene delivery and expression.[note]see note 6[/note] An increase in other immune cells such as CD56+, IL-2 (causing tumor lysis), cytotoxic T lymphocytes, IL-6, TNF-alpha, CD4+ T cells expressing the T cell activation marker CD69, monocytes, and macrophages have been observed.[note]see note 1[/note]
Human and animal studies have shown that WBH can have a profound and therapeutic effect on the immune function of an organism infected with cancer.[note]see note 1, 3-6[/note] The tumor goes into apoptosis with the IR-A heat due to metabolic exhaustion, loss of ATP production, hypoxemia and an increase in lactic acid, production of heat shock proteins; all cause destruction of the cancer.
However, the surrounding healthy tissue is not affected but improved due to the better oxygenation, higher production of ATP, higher blood flow, and the increase and activation of immune cells. The tumor dies within the body, so now the immune system can recognize the cancer cells since the tumor antigens are presented by the invading macrophages to T cells and NK cells.
NK cells are also activated by the production of heat shock proteins. Compared to what conventional oncology has to offer, it is clear that WBH, when done correctly with the right machine, can have a profound impact on cancer and patient survival.
Human Studies
Despite numerous human studies and case reports, WBH is considered experimental, particularly in the US. However, it is used frequently as part of an integrative approach to cancer in Germany and other countries. The vast majority of published data have been on patients with advanced disease as a last resort. In these studies, WBH was used either as a sole or adjunct therapy. The responses achieved with WBH are often remarkable.[note]see note 1[/note]
Cancer multistep therapy was conceived by Dr. Manfred von Ardenne in 1965. It is a combined modality treatment with three process steps: WBH, induced hyperglycemia, and hyperoxemia. It was further developed into a systemic therapy of high efficiency and selectivity, since the knowledge of the synergistic-additive effects of chemotherapy became known.[note]see note 7[/note] This is the manner in which WBH is generally administered in Germany. It will be discussed later.
Thermochemotherapy versus chemotherapy as a choice for advanced metastatic adenocarcinoma
Metastatic adenocarcinomas of the gastrointestinal tract belong to the more difficult tumors in which to slow disease progression. Standard chemotherapy has not significantly improved the long-term survival of these patients. However, WBH with chemotherapy was reported to improve survival. A phase II study was undertaken to compare the effectiveness of WBH plus chemotherapy with chemotherapy alone in patients with treatment-resistant, progressive disease. Nine patients were randomly assigned to a group. All received a continuous infusion of 5FU (425 mg/m2) and leucovorin (20 mg/m2) for 5 days followed by a 24-hour window without therapy, with either WBH (41.8°C for one hour) and chemotherapy or chemotherapy alone.
After 2 monthly cycles, 1 progressive disease and 6 of 7 patients had a partial response who received the combined treatment. In those who received chemotherapy, one remained stable and one progressed. These results indicate that WBH plus chemotherapy is a beneficial treatment choice for metastatic adenocarcinoma.[note]see note 8[/note]
Phase I study: Long-Duration WBH
In another study, fourteen patients with drug-resistant, advanced-bulky or metastatic cancers received 1 to 9 treatment cycles (every 28 days) with gemcitabine and cisplatin and interferon-alpha combined with long-duration WBH (40°C for 6 hours). In 13 patients, 6 objective responses included: 5 partial responses (2 pancreas, 1 gastric, 1 lung, 1 renal), (2/5 PRs were > 90%) and 1 major response with a duration of 5 months (larynx). Additionally, 4 patients had stable disease for longer than 12 weeks. Four patients experienced progressive disease. The authors concluded that that the treatment is safe and well tolerated, and induces meaningful clinical benefit in patients with chemotherapy-resistant tumors.[note]see note 9[/note]
Phase II Clinical Trial for Inoperable or Metastatic Pancreas Cancer
Five patients with inoperable or metastatic pancreatic cancer were treated using a phase II protocol of a sequenced combination of whole body hyperthermia (6 hours at 40°C) with ciplatin (60 mg/m2) and gemcitabine (60 mg/m2) with daily metronomic, low-dose interferon-alpha (1 X106 IU). Patients received 1 to 5 treatments. The researchers documented 2 objective responses (40%), 2 partial responses (40%), and 1 progressive disease (20%) for an 80% overall response. Of interest are that 3 patients who had been heavily pretreated experienced meaningful tumor responses. This regimen appears to demonstrate clinical activity.[note]see note 10[/note]
The authors repeated the protocol with other patients (17 total) and demonstrated a similar clinical response, noting that responding patients experienced an increased quality of life, meaningful responses, and for one patient, clinical remission].[note]see note 11[/note] More than 22 clinical trials have been completed for many types of advanced cancers, using various chemotherapeutic agents as an adjunct. Some studies used higher temperatures (for example, 1 hour at 41.8°C), while other studies used milder temperatures (39.5°C for 3 to 6 hours). The protocols also varied in terms of how many WBH treatments the patients received. More than 9 of these trials are phase II.
Despite the fact that phase III trials have not been completed, clinics abroad, most notably in Germany, regularly use WBH as an integrative approach to cancer care. The reason is that most of these clinics see patients who have advanced disease and/or who have failed treatment, and they have seen firsthand the excellent results achieved using this modality.[note]see note 1[/note] Furthermore, German clinics generally raise the patients’ temperature to 41.6-41.8°C for 60 to 90 minutes — a substantially higher temperature and longer plateau than used in the US. Between the heating and cooling phase, the entire procedure generally lasts 4 to 5 hours.
Experience of St. George Hospital
St. George Hospital is located in Bad Aibling, Germany, approximately one hour from Munich. Since 1991, the clinic exists under the medical direction of Dr. Friedrich Douwes. He is now president of the German Society for Oncology, having been recognized for his outstanding research and clinical contribution to the field of cancer treatment. He has received numerous medical awards for research and clinical work in the field of hyperthermia. In addition to WBH, Douwes employs local-hyperthermia (the tumor is directly heated), transurethral hyperthermia (to induce tumor kill directly to prostate cancer), electrochemical therapy (electrical current is used to induce tumor lysis), immunotherapy (using both natural and prescription immune-stimulating compounds), detoxification (through chelation therapy and colonics), and extensive nutritional support to complement the therapies. However, this discussion will solely focus on the results of WBH.
WBH at St. George Hospital
Douwes and his staff use high-temperature WBH, wherein the body is heated on average 41.6-41.8°C for 90 minutes. The procedure takes 4 to 5 hours to complete, as previously discussed. During the procedure, a temporary state of hyperglycemia using glucose is induced to improve tumor response. Low-dose chemotherapy and/or more natural therapy such as intravenous vitamin C (25-50 grams) are administered during the procedure. The high acidity and hypoxia that occur damage the vessels that nourish the cancer cells. WBH also damages the membranes, proteins, and enzymes of cancer cells, making them more vulnerable to anticancer agents — including herbal, nutritional, chemotherapy, and radiation.[note]see note 12[/note]
Case reports and studies
While Douwes has countless case reports of patients tracked for years, the following is a brief review of some of the success documented in scientific journals, at medical conferences, and from his own files.
Twenty-one patients with recurrent, multidrug-resistant advanced ovarian cancer were treated with WBH and platinum-based chemotherapy. During WBH, a core temperature of 41.5-42°C was achieved. The WBH was combined with artificial hyperglycemia (300-400 mg/dl). The plateau temperature was held over an average of 90±30 minutes and the artificial hyperglycemia on average 240±30 minutes. The treatment was repeated every 3 to 4 weeks for several months. One patient (4.8%) had complete remission, 7 patients (33.3%) had partial remission, stable disease was noted in 10 patients (47.6%), and 3 (14.3%) did not respond and had progressive disease. Median time to progression was 6.4 months and median survival time was 16.5 months. For the responder group (complete and partial remission), the median survival time was 21.5 months.
Three to four days afterward, WBH tumor symptoms such as loss of appetite, weakness, and pain subsided remarkably. Pain-relief medications were able to be reduced, and for some patients ceased altogether. Patients with a longer treatment-free interval (more than 24 months) showed better outcome. The response rate was 60% and the median survival time was 33 months compared with a 28.6% response rate and a median survival time of 14 months in patients with treatment-free intervals of 6 to 12 months. It is important for the assessment of the clinical results to note that the majority of patients had multiple pretreatments with various cytostatics, had failed treatment, and had become multidrug resistant.
Other studies have shown excellent response rates for ovarian cancer as well as for other types of cancer such as sarcoma.[note]see note 13[/note]
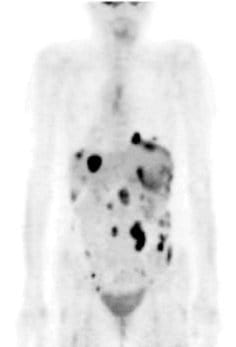
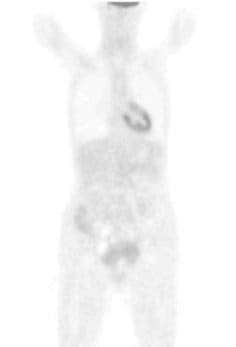
Case report: SM
An example of an excellent clinical case is SM, a 47-year-old woman diagnosed with stage 3C metastasizing adenocarcinoma of the ovary in August 2005. She had previously had her ovaries removed, after which time she received 6 cycles of chemotherapy with taxol and carboplatin, beginning December 8, 2005. She experienced a continuous increase in tumor markers; and a CT scan July 1, 2005, revealed metastatic spread. She received further chemotherapeutic treatment with Caelyx (liposomal, encapsulated doxyrubicin) and did not achieve any results. The therapy was aborted; and she was told to get her affairs in order, since she had only a few months to live. She was seen for the first time at St. George Hospital on August 22, 2007. She received 50 mg of cisplatin intraperitoneally with simultaneous systemic mitomycin C 10 mg and 20 mg liposomal doxorubicin (Doxil).

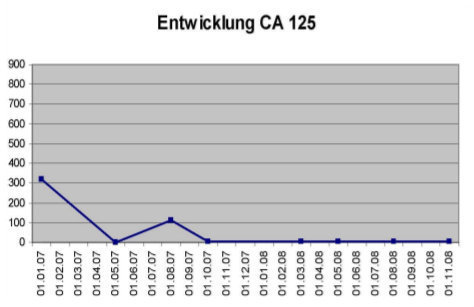
Her core temperature was elevated to 41.6°C for 120 minutes. She also received locoregional hyperthermia to her abdomen 3 times per week at a temperature of 42-44°C, as well as immunotherapy (e.g., thymus extract), nutritional support, intravenous vitamin C (25 grams), and detoxification (chelation therapy). On August 29, 2007, she received the same protocol. On October 24 and 31, 2007, again she received the same WBH protocol without the intraperitoneal infusion. Peritoneal infusion was no longer necessary, since there was no longer any ascites. She was in remarkably improved condition, with no pain and normal bowel function. Her initial CA-125 had been 112U/I and it was now down to 4.5 U/I. The patient received her final treatments on March 12 and 19, 2008, with the same systemic protocol. Prior to the treatment, her PET scan was negative. Her CA-125 was now down to 3.7 U/I. She remains alive and well today.[note]see note 14[/note]
Case report: RF
Another case is RF, a 60-year-old woman diagnosed with breast cancer in 1986 (moderate differentiated invasive ductal, pT2aN0MxG2L1). She had conventional treatment with removal of the right breast, followed by 3 cycles of chemotherapy (with FAC schedule; polychemo treatment). She then stopped because she could not tolerate the chemotherapy and was put on Tamoxifen. In 1989 (3 years later), she had a local recurrence that was removed by surgery. In 1992, she again had a local recurrence, although she was under antihormonal therapy with Tamoxifen. She was then treated by radiotherapy (50, 4 Gray). After this treatment she developed a lymphedema in her right arm. In 1994 she had again a local relapse in the right thoracic wall, this time also with involvement of her lymph nodes in the right supraclavicular area. She refused any further conventional treatment and went to St. George Hospital. RF received 4 whole body hyperthermias (initial treatment July 8, 1996) in combination with 10 local hyperthermias with low-dose chemotherapy (FEM schedule, 50 mg Epirubicin, 30 mg Mitomycin, 750 mg 5-FU) (5-FU, Epirubicin, Mitomycin). She received one whole body hyperthermia treatment each week for 4 weeks (7 days apart) and local hyperthermia 3 or 4 times per week. This brought her into a long-lasting remission. The after-care follow-up was negative up to her last visit in December 2008. She is on a complementary medicine maintenance program.[note]see note 12[/note]
Review article: Hyperthermia and Electrotherapy
An excellent review article was written by H. Kaltsas in 2000, titled “Too Hot for Cancer: Hyperthermia and Electrotherapy.” Several of Douwes’s patients were interviewed, including a patient with late-stage terminal ovarian cancer that after 8 years had spread to her liver, colon, and bladder. After several WBH treatments with low-dose chemotherapy and locoregional hyperthermia, the patient was in excellent health. She returns to the clinic once or twice a year to keep up the remission. Also a 56-year old man with extensive non-Hodgkin’s lymphoma visible throughout his body received several WBH treatments with excellent results. In this way he was able to tolerate the chemotherapy, since with WBH the dose used was 60% less than what is normally used.[note]see note 15[/note]
Douwes has excellent case studies of advanced breast, colon, lung, sarcoma, and many other types of cancer that have responded remarkably to WBH along with an integrative approach. Perhaps that is why he is achieving even greater success than what is being reported elsewhere.
A Different Philosophy
There is a fundamentally different philosophy about the cancer treatment at St. George Hospital. Douwes is not going to wait for phase III trials to confirm what he already knows about WBH. Furthermore, most of the published clinical trials using WBH have already shown remarkable results.[note]see note 1-11,13[/note]
Douwes has practiced oncology since 1975. For long, he was unsatisfied with the results of the conventional approach to cancer. It is clear that the “war on cancer” has not been won with the vast majority of conventional therapies available. This is what led him on the path to finding the very best modalities that can add meaningful quality time to the life of a cancer patient. He was also not pleased with patients’ being “scared to death into treatment”: being told they will die if the malignancy is not treated immediately and rushed into therapy (whether the research shows it works or not) without time to explore other options. Douwes prefers to have a more positive attitude with patients; so many come to him already hopeless, it is important that they know they still have a chance.
As previously mentioned, the treatment protocols at St. George Hospital include nutritional supplementation, injections, and intravenous infusion of a variety of compounds to suit the needs of the patient. The clinic also has electrochemical therapy, locoregional hyperthermia, trans-urethral hyperthermia (to treat prostate cancer), and a combination of electro and heat therapy that can be applied with a long needle directly into metastasis of the liver or a breast tumor to avoid the complications of surgery.
One of the main philosophical differences is that, while clinical remission is often achieved, there is always the chance that a cancer can return at a later date. Also, patients are generally told that they are “cured” immediately after conventional treatment. This is untrue, since the cancer often comes back more even vehemently, they acquire a secondary cancer from their treatment, and/or the cancer becomes multidrug resistant.
Maximum Success and Lifespan
There are many patients who are kept alive and well for years (or indefinitely as of now) because they keep up with their treatments at St. George Hospital 1 or 2 times per year (on average). While a complete remission may not have been achieved for some of these patients, their cancer is kept at bay. Instead of feeling like “walking time bombs,” patients are encouraged to keep up with their programs to achieve maximum success and lifespan.
We hope that one day the type of treatment offered at Klinik St. Georg will be considered mainstream. In this case, patients will be more likely to be treated at the clinic (and elsewhere) at the beginning rather than near the end of their disease and even better results will be achieved. They would be coming with cancer diagnosed at an earlier stage and they would not have been given many rounds of chemotherapy (and/or radiation) treatment and failed.
Of course, Douwes has also had patients coming to the clinic for the first line of treatment. But that is not generally the case. Most patients who come from abroad including the US, still do so after they have failed all conventional treatments. Despite this, Douwes had achieved outstanding results with these patients. If people can live with heart disease or diabetes, there is no reason why someone cannot live a full and productive life with cancer, especially if they take care of their lifestyles and find successful treatment options.
Notes
- Rowe-Horwege RW. Hyperthermia, systemic. In: Webster JG, ed. Encyclopedia of Medical Devices and Instrumentation, 2nd ed. John Wiley and Sons Inc.: 2006.
- Dayanc BE, Beachy SH, Ostberg JR et al. Dissecting the role of hyperthermia in natural killer cell mediated anti-tumor response. Intl Hyperthermia. 2008:24 (1):41-56.
- Xu Y, Choi J, Hylander B, et al. Fever-range whole body hyperthermia increases the number of perfused tumor blood vessels and therapeutic efficacy of liposomally encapsulated doxorubicin. Intl Hyperthermia. 2007:23 (6): 513-527.
- Manjili MH, Wang XY, Macdonald IJ et al. Cancer immunotherapy: stress proteins and hyperthermia. Int J Hyperthermia. 2002:18 (6): 506-520.
- Appenheimer MM, Chen Q, Girard RA, et al. Impact of fever-range thermal stress on lymphocyte-endothelial adhesion and lymphocyte trafficking. Immunol invest. 2005:34: 295-323.
- Strebel FR, Deng W, Loose DS, et al. Hyperthermic enhancement of systemic liposomal gene delivery and expression in tumors. 9th International Congress on Hyperthermic Oncology; April 20-24, 2004; St. Louis, MO. Posters. Biology. 119.
- Von Ardenne M. Pri nci ples and concept 1993 of the Systemic Cancer Multistep Therapy (sCMT). Extreme whole body hyperthermia using the infrared-A technique IRATHERM 2000 — selective thermosensitisation by hyperglycemia —circulatory back up by adapted hyperoxemia. Strahlenther Onkol. 1994:170 (10): 581-589.
- Nagle V, Bull J, Berry J. Thermochemotherapy versus chemotherapy as a choice for advanced metastatic adenocarcinoma [abstract P01-16]. Forty-Sixth Annual Meeting of the Radiation Research Society and the Seventeenth Annual Meeting of the North American Hyperthermia Society Joint Meeting; April 25-29, 1998; Louisville, KY.
- Bull JM, Nagle C, Lee V, et al. A phase I study of optimally-timed gemcitabine (GEM) + cisplatin (Cis)/interferon-alpha (IFN) combined with long-duration, low temperature whole body hyperthermia [abstract]. International Clinical Hyperthermia Society XXVII Convention; January 6-7, 2007; Mum bai, India.
- Bull JM, Scott GL, Graham JL, et al. A new phase II clinical trial for inoperable or metastatic pancreas cancer using fever range whole body thermal therapy (FR-WB-TT) + ciplatin (CIS) + gemcitabine (GEM) + metronomic lose dose interferon-alpha [abstract]. Society of Thermal Medicine Annual Meeting; April 1-3, 2005; Bethesda, MD.
- Bull JM, Scott GL, Figueroa G. et al. An update of Phase II clinical trial using fever range whole body thermal therapy (FR-WB-TT) + ciplatin (CIS) + gemcitabine (GEM) + metronomic low dose interferon-alpha for inoperable or metastatic pancreas cancer (abstract). International Clinical Hyperthermia Society XXVII Convention; January 6-7, 2007; Mumbai, India.
- Douwes F. Personal communication; November 15, 2008.
- Douwes F, Bovic J, Douwes 0, et al. Whole-body hyperthermia in combination with platinum-containing drugs in patients with recurrent ovarian cancer. Intl Clin Oncol. 2004:9: 85-91.
- Douwes F. Documented case studies. Private communication.
- Kaltsas H. Too hot for cancer: hyperthermia and electrotherapy. Ahern Med. 2007:37: 1-8.
This article by Shari Lieberman, PhD, CNS, FACN appeared in Aug/Sept 2009 Townsend Letter.



