Abstract
Background: Patients with advanced ovarian cancer have an enormous risk of relapse after primary therapy, and the prognosis for these patients remains bleak. Primary and acquired resistance of tumor cells to antineoplastic drugs is a major cause of the limited effectiveness of chemotherapy. The effect of whole-body hyperthermia (WBH) combined with platinum-containing chemotherapy in the treatment of recurrent ovarian cancer was examined in this study.
Methods: Patients studied were those with pathologically verified epithelial ovarian cancer after operation who had had first-line chemotherapy with cisplatin or carboplatin, and relapsed. All 21 patients were treated with WBH and platinum-based chemotherapy. During the WBH, a core temperature of 41.5°C-42°C was attained in the rectum. We combined the WBH with 300-400 mg/di artificial hyperglycemia. The plateau temperature was held over a period of, on average, 90 ± 30 min, and the artificial hyperglycemia, on average, 240 ± 30 min. WBH was repeated at the beginning of each new chemotherapy cycle.
Results: One patient (4.8%) had a complete remission, 7 patients (33.3%) had a partial remission, stable disease was noted in 10 patients (47.6%), and 3 (14.3%) patients did not respond and had progressive disease. Median time to progression was 6.5 months, and median survival time, 16.5 months.
Conclusion: Our results validate the efficacy of WBH in the treatment of patients with recurrent platinum-resistant ovarian cancer. The overall tolerance of this treatment was good. The priority for all patients was an improvement in life quality; this was seen 3-4 days after WBH. The encouraging results should be confirmed in randomized studies.
Key words: Carboplatin, Cisplatin, Ovarian cancer, Whole-body hyperthermia
1. Introduction
Of all gynecological malignancies, ovarian cancer is the commonest cause of death in developed countries. Because ovarian cancer is often asymptomatic in the early stages, most patients have widespread disease at the time of diagnosis. In most cases, the diagnosis does not take place until advanced tumor stages, International Federation of Gynecology and Obstetrics (FIGO) III and IV. Combined platinum-based chemotherapy has become the standard initial treatment for advanced ovarian cancer. Despite high overall and complete clinical response rates, in most of these patients disease recurs or becomes resistant to the drug used during the primary induction chemotherapy. In the salvage therapy of platinum-pretreated patients with a disease-free interval of more than 6 months, renewed treatment with platinum should be considered.[note]see reference 1[/note] Unfortunately, available treatment regimens for recurrent ovarian cancer have yielded disappointing results, with the majority of responses also being of short duration. Thus, it is necessary to examine the impact of new treatment modalities to improve response rates and survival of ovarian cancer patients with recurrent disease. The primary cause of the limited effectiveness of second- or third-line chemotherapy in the treatment of recurrent carcinoma is the multi-drug resistance (MDR) of cancer cells to antineoplastic drugs. To overcome MDR, either the effectiveness of the drugs used or the vulnerability of the cancer cells must be enhanced.
Heat represents such a method to improve the effect of cytostatic therapy. Different forms of hyperthermia have been used in the treatment of cancer for over two decades, demonstrating its effectiveness in combination with both radio- and chemotherapy. The increased temperature and the resulting activation of cell metabolism induces hypoxia, ATP depletion, and acidosis of the tumor tissue.[note]see references 2-5[/note] Hyperthermia causes disturbances in the microcirculation of cancer tissue,[note]see reference 6[/note] results in an inhibition of the DNA repair mechanisms,[note]see references 7, 8[/note] and induces apoptosis.[note]see reference 9[/note] The treatment modulates the activity of cytokines[note]see references 10, 11[/note] and increases the antigenicity of tumor cells by the production of heat shock proteins and the activation of natural killer cells.[note]see references 12, 13[/note] The results in the past decades have demonstrated that an increase in temperature can dramatically enhance the antineoplastic effects of cytostatics. It can be demonstrated that there is not only a relation between dose and response, but also between temperature and the response of a therapeutic substance. The cytotoxic activity of such antineoplastic drugs as cyclophosphamide, carboplatin, and cisplatin is increased.[note]see references 14,15[/note] There are a number of factors causing the temperature-dependent enhancement of the cytotoxicity. Blood circulation and the transport of drugs to the tumor are increased by the first phase of hyperthermia. Studies have shown that the concentration of cytostatics inside the tumor is enhanced[note]see reference 16[/note] and that biochemical reactions of the antineoplastic substances are temperature-dependent; for example, the formation of DNA adducts by platinum-containing drugs.[note]see reference 14[/note]
Whole-body hyperthermia (WBH) can be successfully applied for the treatment of advanced metastatic cancer. Depending on the body temperature reached during a hyperthermia treatment, we can differentiate between moderate (less than 41.5°C) and extreme (41.5°C-42.0°C) WBH. Most studies with WBH in anticancer treatment were performed with extreme WBH, but reports of moderate WBH with a duration of up to 6h show that it may also be a feasible approach. Several different techniques have been developed, with the same goal – to increase the core temperature.
Heating by supplying external energy can be performed with infrared radiation, which is, apart from extracorporal heating, used in most contemporary procedures. The main difference between the WBH techniques is in the length of the infrared waves. Longwave infrared hyperthermia (wavelength of 8µm) can be applied for heating, using water-filled tubes with a temperature of 60°C. The patient is positioned in a moisture-saturated chamber; the superficially adsorbed energy is transported by the increased skin blood circulation. Using radiators with a temperature of 2400°C, shortwave infrared A (wavelength, 0.75-1.4µm) radiation, which is filtered using water or metal reflectors, can be emitted. The adverse effects have been decreased with this method, because this type of filtered infrared radiation results in a higher penetration depth and in a more regular heating of the tissue.[note]see reference 17[/note]
An artificial increase in the glucose level induces additional acidosis and causes a synergistic cytotoxic effect.[note]see reference 18[/note]
Many clinical trials have been performed to study the effects of hyperthermia in cancer therapy. Results of phase III trials showed increased response and survival time for many types of cancer:[note]see references 19-22[/note] Earlier phase I/II studies demonstrated safety and increased response rates of WBH with platinum-based cytostatics.[note]see references 23, 24[/note]
The aim of this retrospective analysis was to show how the combination of WBH and platinum-based chemotherapy can improve the response rate, survival time, and time to progression in patients with advanced ovarian cancer who relapsed after primary chemotherapy with platinum.
2. Patients and methods
2.1 Patients
Twenty-one patients with recurrent epithelial ovarian cancer were enrolled in our study. All had histologically proven ovarian cancer, confirmed according to FIGO. Patients were selected according to the following criteria: all patients were suffering from recurrent ovarian cancer after previous surgery and first-line chemotherapy with platinum-based cytostatic drugs, with a treatment-free interval of at least 6 months. The condition for a retrospective analysis was a documented and measurable lesion, proved by imaging, such as computed tomography (CT), magnetic resonance 1.imaging (MRI), X-ray, or sonogram. Patients were excluded from treatment with hyperthermia if they suffered from brain metastasis, cardiopulmonary or renal failure, severe diabetic microangiopathy, or hypersensitivity to medication included in our treatment. A written informed consent document was obtained from each patient. Six patients received WBH therapy as a second-line therapy, and 15 patients received the therapy after previous extensive multiple chemotherapies (Table 1). The median number of prior chemotherapies was 3 (range, 1-9). The number of organs with evidence of disease was as follows: metastases in one organ in 5 subjects (23.8%); two organs in 7 subjects (33.3%); and three organs in 9 subjects (42.9%). Median age at the beginning of combination therapy was 54.5 years (range, 34.1-66.5 years) (Table 1).
2.2 Treatment regimen
The patients received chemotherapy and WBH in combination. Either cisplatin or carboplatin was given, at dosages of 50 mg/m2 and 400 mg/m2, respectively. Cisplatin (Hexal, Holzkirchen, Germany) or carboplatin (Hexal) was administered during the first 30 min of WBH. During this initial phase of therapy (modest-temperature hyperthermia) blood flow to the tumor is enhanced, thereby increasing the concentration of the chemotherapeutic agents in the cancerous tissue.[note]see reference 16[/note] WBH in our hospital was carried out with special units emitting infrared A light (Whole-Body Hyperthermia Unit; Heckel — HT2000 73728 Esslingen, Germany; electrical data, four infrared emitters at 300W; color temperature, 2450 K; wavelength maximum emission, 1.180 µm — infrared A). Body temperature was monitored in the rectum, bladder, tympanum, and skin/muscle. During the WBH, a core temperature of 41.5°C-42°C was obtained, and patients were kept at this plateau for 90 ± 30 min. The WBH was carried out with intravenous sedation, using a mixture of morphine, midazolam, propofol, and fentanyl; general anesthesia was not necessary. The head was cooled once a temperature of 40°C had been reached. To establish additional acidosis of the tumor tissue, artificial hyperglycemia was induced by an i.v. infusion of 20% glucose, with an increase of the blood sugar level to 300-400 mg/di. This hyperglycemia was continued during the entire plateau phase of WBH, on average for 240 ± 30 min. During the entire procedure, vital functions, including heart rate, blood pressure, and blood oxygen, were monitored; and fluid, electrolytes, and blood gases were balanced if necessary. After the end of WBH, patients were monitored for 24h in our intensive care unit for safety. The treatment was repeated every 3 to 4 weeks.
Table 1: Individual data and therapy results of patients entering the retrospective analysis of combined therapy with WBH and chemotherapy
| No. | Age (years) | Organs with metastasis | Karnofsky index | Survival since first diagnosis (months) | Prior chemotherapies | No. of prior chemotherapies |
|---|---|---|---|---|---|---|
| 1 | 46.1 | 3 | 100 | 54 | CBDCA, CTX, TAX, TOPO | 4 |
| 2 | 34.1 | 3 | 90 | 42 | CDDP, TAX | 1 |
| 3 | 55.3 | 1 | 100 | 106 | CDDP, CTX, CBDCA, TAX, TREO, GEM | 5 |
| 4 | 40.3 | 2 | 100 | 36 | CBDCA, TAX | 1 |
| 5 | 66.5 | 2 | 80 | 35 | CBDCA, TAX | 1 |
| 6 | 60.2 | 2 | 80 | 117 | CDDP, TREO, CBDCA, ETO, TAX | 3 |
| 7 | 58.0 | 2 | 80 | 120 | CDDP, CTX, TAX | 2 |
| 8 | 54.0 | 3 | 90 | 41 | CBCDA, TAX, TREO | 2 |
| 9 | 60.9 | 3 | 100 | 48 | CBDCA, CDDP, TAX, TREO | 4 |
| 10 | 53.9 | 1 | 90 | 50 | CDDP, CTX, TAX, TREO | 5 |
| 11 | 48.9 | 3 | 100 | 56 | CBDCA, CDDP, ETO, TREO, 5-FU | 4 |
| 12 | 61.2 | 3 | 80 | 98 | CDDP, CTX | 1 |
| 13 | 57.9 | 90 | 52 | CDDP, TREO | 5 | |
| 14 | 54.5 | 3 | 90 | 79 | CBDCA, CTX, TAX, TREO, GEM | 4 |
| 15 | 41.6 | 2 | 80 | 56 | CBDCA, CTX, TAX | 2 |
| 16 | 41.1 | 2 | 80 | 58 | CBDCA, TAX, GEM | 3 |
| 17 | 62.0 | 2 | 80 | 35 | CDDP, CTX, CBDCA, TAX, CTX | 1 |
| 18 | 59.2 | 1 | 90 | 40 | 2 | |
| 19 | 58.5 | 3 | 90 | 189 | CDDP, DOX, CTX, CBDCA, NVB, ETO, GEM | 5 |
| 20 | 52.2 | 1 | 80 | 35 | CDDP, TAX | 1 |
| 21 | 42.8 | 3 | 100 | 92 | CBDCA, CTX, TAX, TOPO, TREO, GEM, NVB | 9 |
2.3 Clinical response and assessment
Only patients with measurable lesions were included in our analysis. Responses were assessed according to the following definitions. A complete remission (CR) meant the disappearance of all evidence of measurable and assessable tumor disease, and normalization of tumor markers and laboratory values for at least 4 weeks. A partial response (PR) was designated as greater than or equal to a 50% decrease in the sum of the products of the perpendicular diameters of all measured lesions for at least 4 weeks. No lesions should increase in size and no new lesion should appear. Stable disease (SD) was defined as a steady state or a response less than a PR, but with no disease progression for at least 4 weeks. No new lesions could appear and no symptoms could worsen. Progressive disease (PD) was defined as any increase of 25% or more in the sum of the products of the perpendicular diameters of any measurable lesion. Time to progression and overall survival time were measured from the beginning of therapy to the time of disease progression and time of death, respectively. The Kaplan-Meier method was used to calculate survival probabilities for all patients.
3. Results
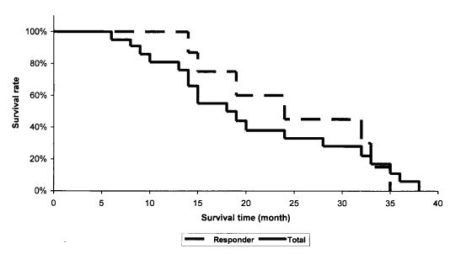
The overall response rate was 38.1%; 1 patient had a complete remission (CR), and 7 patients (33.3%) had a partial remission of their ovarian cancer lesions. Stable disease was observed in 10 patients (47.6%), and 3 patients (14.3%) had progressive disease. Life tables of the patients were obtained and survival curves were computed using the Kaplan-Meier method. Median survival time from first diagnosis was 53 months (range, 35 to 189 months). Median survival time, measured from the beginning of the combination therapy in all patients, was 16.5 months (range, 6 to 38 months). For the responder group (complete and partial remission) the median survival time reached 21.5 months (Fig. 1). Median time to progression for all patients was 6.5 months (range, 2-24 months).
The combination therapy used carboplatin (15 patients) or cisplatin (6 patients) for the platinum-based cytotoxic therapy and WBH. The outcome of treatment with carboplatin was an overall response rate of 33.3% (5 patients); 8 patients (53.3%) showed stable disease, and 2 (13.3%), progressive disease. The median survival time was 15 months. In the cisplatin-treated group, 3 patients (50.0%) showed remission, 2 had stable disease, and 1 patient, showed progressive disease. The median survival time was 17 months (Table 2).
Table 2: Response to combination therapy using carboplatin or cisplatin
| Therapy | n | Complete remission | Partial remission | No change | Progressive disease | Survival (months) |
|---|---|---|---|---|---|---|
| Carboplatin | 15 | 1 | 4 | 8 | 2 | 15 |
| Cisplatin | 6 | 3 | 2 | 1 | 17 |
For assessment of the effect of the treatment-free interval on the results of renewed chemotherapy with platinum-containing substances, the patients were divided into three groups. Seven patients had a short treatment-free interval, of 6-12 months. Their response rate was 28.6%, and the median survival time, 14 months. The nine patients with a treatment-free interval of 12-24 months had a remission rate of 33.3% and a median survival of 15 months. Five patients received no cytostatic treatment for a period of more than 24 months. In this group, one complete and two partial remissions were observed (60.0% overall remission rate), and the median survival time was 33 months (Table 3).
Table 3: Effect of the treatment-free interval on the outcome of the renewed platinum-based therapy
| Treatment-free interval | n | Complete remission | Partial remission | No change | Progressive disease | Survival (months) |
|---|---|---|---|---|---|---|
| 6-12 | 7 | 0 | 2 | 3 | 2 | 14 |
| 12-24 | 9 | 0 | 3 | 5 | 1 | 15 |
| >24 | 5 | 1 | 2 | 2 | 0 | 33 |
The priority for all patients was an improvement in life quality. Three to four days after the WBH, the subjective status of the majority of the patients was significantly improved; the tumor symptoms had subsided remarkably (e.g. loss of appetite, weakness, pain, etc). We were able to reduce pain relief medication and, in certain patients, we were able to cease pain relief medication. Clearly perceptible reduction of ascites was observed in four of the five patients with ascites.
3.1 Toxicity
Myelosuppression was the major toxic effect and was induced by the cytostatics used. Six patients suffered from leucopenia grade 1, three patients had grade 2, and three patients, grade 3 (Table 4). Thrombocytopenia grade 1 appeared in three patients, and grade 2 also in three patients. In one patient, paresthesia occurred after treatment with hyperthermia and carboplatin. Emesis grade 3 appeared in three patients; carboplatin was used in one patient and cisplatin in two. No cardiovascular or kidney toxicities were observed. The side effects of hyperthermia were mild; patients showed skin reactions in the form of a mild burn, grade 1.
Table 4: Individual data and results of therapy given to patients
| No. | No. of therapies | Maximum temperature during WBH (CO) | Response | Survival (months) | Time to progression (months) | Leucopenia (NCI grade) |
|---|---|---|---|---|---|---|
| 1 | 4 | 41.5 | NC | 9+ | 7 | 2 |
| 2 | 3 | 41.8 | NC | 10 | 6 | 0 |
| 3 | 4 | 41.9 | PR | 15+ | 7 | 2 |
| 4 | 6 | 41.9 | NC | 8 | 2 | 0 |
| 5 | 3 | 41.9 | NC | 13 | 4 | 0 |
| 6 | 3 | 42.0 | PR | 33 | 9+ | 1 |
| 7 | 4 | 42.0 | NC | 18 | 3 | 1 |
| 8 | 4 | 41.2 | PD | 20 | 0 | |
| 9 | 1 | 41.7 | NC | 14 | 8 | 0 |
| 10 | 4 | 41.8 | PR | 14 | 3+ | 0 |
| 11 | 1 | 41.8 | NC | 6 | 3 | 1 |
| 12 | 2 | 41.8 | CR | 35 | 15 | 3 |
| 13 | 2 | 41.9 | PD | 15 | 0 | |
| 14 | 5 | 41.4 | NC | 38 | 24 | 0 |
| 15 | 5 | 41.6 | PR | 32 | 11 | 0 |
| 16 | 4 | 41.8 | PR | 24 | 6+ | 1 |
| 17 | 2 | 41.9 | PD | 9 | 3 | |
| 18 | 4 | 41.6 | PR | 15 | 2 | 1 |
| 19 | 1 | 42.0 | NC | 36 | 4+ | 3 |
| 20 | 5 | 41.6 | PR | 19 | 7 | 1 |
| 21 | 4 | 41.9 | NC | 28 | 12 | 2 |
complete remission; NCI, National Cancer Institute
4. Discussion
In the first-line therapy of advanced ovarian cancer, the highest remission rates can be achieved through the use of platinum-containing drugs.[note]see reference 25[/note] However, most patients relapse and, in this case, the time to progression tends to be brief, and the side effects of the salvage therapy are significant.[note]see reference 26[/note]
The response rate in platinum-based second-line therapy after a failed first-line treatment with platinum-containing cytostatics is dependent on the duration of the therapy-free interval, and ranges from 13% to 33%.[note]see reference 1.27-29[/note] Median survival time after salvage chemotherapy of recurrent ovarian cancer observed in clinical studies was 7-12 months..[note]see reference 27-29[/note] In view of this modest result, new therapy modalities have to be investigated.
Chemosensitivity to the drugs plays a crucial role in the treatment of ovarian cancer. Successful chemotherapy for human cancers is apparently limited by intrinsic or acquired MDR towards cytostatic drugs. The extremely complex molecular mechanisms of the sensitivity and resistance of tumor cells to chemotherapy are only partially understood. A panel of MDR-associated genes and proteins respond to coordinated treatment with effective modulating agents. Cytokines and immunological agents, as well as gene therapy, can affect the expression of several MDR-associated genes. But only a detailed investigation of all the different types of MDR may provide the chance to fully understand these multifactorial events and the chance to develop complex strategies for overcoming drug resistance. Hyperthermia in combination with chemotherapy has a strong biological rationale, based on thermal enhancement of cytotoxicity and partial circumvention of resistance. Effects of hyperthermia on ovarian cancer were described in many preclinical and clinical studies. In vitro experiments showed enhanced effectiveness of antineoplastic substances and an overcoming of resistance to platinum drugs in ovarian cells, caused by heat injures to DNA, proteins, lipids, and enzyme conformations, and cell functions, with the synergistic induction of apoptosis.[note]see reference 14, 30 31[/note] The thermal enhancement ratio of cisplatin, which represents the ratio of the dose causing cell death in the presence or absence or hyperthermia, was up to 3.8, measured in platinum-resistant cells.[note]see reference 32[/note] In a pilot study, a combination therapy of WBH and carboplatin treatment in patients with platinum-resistant ovarian cancer had a remarkable outcome.[note]see reference 33[/note]
In our study, we examined patients suffering from recurrent epithelial ovarian cancer after first-line treatment with platinum-containing drugs. The patients received salvage chemotherapy with platinum-containing drugs combined with WBH. We observed a response rate of 38.1%, only 14.3 % of patients with progressive disease, and a median survival of 16.5 months. Patients with a longer treatment-free interval (more than 24 months) showed a better outcome. The response rate was 60% and the median survival time 33 months, compared to a 28.6% response rate and a median survival of 14 months in patients with a treatment-free interval of 6-12 months. It is important for assessment of the clinical results that the majority of the treated patients had had multiple pretreatments with various cytostatics.
The retrospective analysis shows encouraging results in the treatment of relapsed ovarian cancer. Whole-body hyperthermia can make a contribution to overcome drug resistance and to increase the response to retreatment with cisplatin or carboplatin, even after multiple prior chemotherapies. Previous studies have shown excellent response rates with the utilization of WBH and chemotherapy for ovarian cancer,[note]see reference 33[/note] as well as for other types of cancer, such as sarcoma. [note]see reference 34-36[/note] Our study also demonstrated high response rates with two to three WBH treatments in combination with chemotherapy. Further studies, in particular randomized phase III trials, are required to see if more WBH treatments would be able to improve response rates in treatment-resistant patients.
References
- Markman M, Rothman R, Hakes T, et al. (1991) Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 9:389-393.
- Bicher HI, Hetzel FW, Sandhu TS, et al. (1980) Effects of hyperthermia on normal and tumour microenvironment. Radiology 137:523-530.
- Schaefer C, Mayer WK, Kruger W, et al. (1993) Microregional distributions of glucose, lactate, ATP and tissue pH in experimental tumours upon local hyperthermia and/or hyperglycaemia. J Cancer Res Clin Oncol 119:599-608.
- Vaupel P, Ostheimer K, Muller-Klieser W (1980) Circulatory and metabolic responses of malignant tumours during localized hyperthermia. J Cancer Res Clin Oncol 98:15-29.
- Vujaskovic Z, Poulson JM, Gaskin AA, et al. (2000) Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys 46:179185.
- Bogovic J, Douwes F, Muravjov G, et al. (2001) Posttreatment histology and microcirculation status of osteogenic sarcoma after a neoadjuvant chemo- and radiotherapy in combination with local electromagnetic hyperthermia. Onkologie 24:55-58.
- Li Q, Bostick-Bruton F, Reed E (1998) Effect of interleukin-1 alpha and tumour necrosis factor-alpha on cisplatin-induced ERCC-1 mRNA expression in a human ovarian carcinoma cell line. Anticancer Res 18:2283-2287.
- Osman A el-M, Ahmed MM, Khayyal MT, et al. (1993) Hyperthermic potentiation of cisplatin cytotoxicity on solid Ehrlich carcinoma. Tumori 31;79:268-272.
- Sakaguchi Y, Stephens LC, Makino M, et al. (1995) Apoptosis in tumours and normal tissues induced by whole body hyperthermia in rats. Cancer Res 55:5459-5464.
- Katschinski DM, Wiedemann GJ, Longo W, et al. (1999) Whole body hyperthermia cytokine induction: a review, and unifying hypothesis for myeloprotection in the setting of cytotoxic therapy. Cytokine Growth Factor Rev 10:93-97.
- Neville AJ, Sauder DN (1988) Whole body hyperthermia (4142°C) induces interleukin-1 in vivo. Lymphokine Res 7:201206.
- Multhoff G (1997) Heat shock protein 72 (HSP72), a hyperthermia-inducible immunogenic determinant on leukemic K562 and Ewing’s sarcoma cells. Int J Hyperthermia 13:39-48.
- Roigas J, Wallen ES, Loening SA, et al. (1998) Heat shock protein (HSP72) surface expression enhances the lysis of a human renal cell carcinoma by IL-2 stimulated NK cells. Adv Exp Med Biol 451:225-229.
- Hettinga JV, Lemstra W, Meijer C, et al. (1997) Mech.anism of hyperthermic potentiation of cisplatin action in cisplatin-sensitive and -resistant tumour cells. Br J Cancer 75:1735-1743
- Wiedemann G, Roszinski S, Biersack A, et al. (1992) Local hyperthermia enhances cyclophosphamide, ifosfamide and cisdiamminedichloroplatinum cytotoxicity on human-derived breast carcinoma and sarcoma xenografts in nude mice. J Cancer Res Clin Oncol 118:129-135.
- Wiedemann GJ, Siemens HJ, Mentzel M, et al. (1993) Effects of temperature on the therapeutic efficacy and pharmacokinetics of ifosfamide. Cancer Res 53:4268-4272.
- Wehner H, von Ardenne A, Kaltofen S (2001) Whole-body hyperthermia with water-filtered infrared radiation: technical-physical aspects and clinical experiences. Int J Hyperthermia 17:19-30.
- Steinhausen D, Mayer WK, von Ardenne M (1994) Evaluation of systemic tolerance of 42.0 degrees C infrared-A whole-body hyperthermia in combination with hyperglycemia and hyperoxemia. A phase-I study. Strahlenther Onkol 170:322-334.
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, et al. (1995) Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 345:540-543.
- Vernon CC, Hand JW, Field SB, et al. (1996) Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 35:731-744.
- Valdagni R, Amichetti M (1994) Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys 28:163169.
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, et al. (2000) Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 355:1119-1125.
- Robins HI, Cohen JD, Schmitt CL, et al. (1993) Phase I clinical trial of carboplatin and 41.8°C whole-body hyperthermia in cancer patients. J Clin Oncol 11:1787-1794.
- Wiedemann GJ, d’Oleire F, Knop E, et al. (1994) Ifosfamide and carboplatin combined with 41.8°C whole-body hyperthermia in patients with refractory sarcoma and malignant teratoma. Cancer Res 54:5346-5350.
- Aabo K, Adams M, Adnitt P, et al. (1998) Chemotherapy in advanced ovarian cancer: four systematic meta-analyses of individual patient data from 37 randomized trials. Advanced Ovarian Cancer Trialists’ Group. Br J Cancer 78:1479-1487.
- Roland PY, Barnes MN, Niwas S, et al. (1998) Response to salvage treatment in recurrent ovarian cancer treated initially with paclitaxel and platinum-based combination regimens. Gynecol Oncol 68:178-182.
- Ghamande SA, Piver MS (1999) Role of salvage chemotherapy with topotecan and cisplatin in patients with paclitaxel- and platinum-resistant recurrent ovarian or primary peritoneal cancer: a phase II pilot study. J Surg Oncol 72:162-166.
- van der Burg ME, Hoff AM, van Lent M, et al. (1991) Carboplatin and cyclophosphamide salvage therapy for ovarian cancer patients relapsing after cisplatin combination chemotherapy. Eur J Cancer 27:248-250.
- Dobbs SP, Gribbin C, Chan SY, et al. (1994) Second-line treatment with ifosfamide and carboplatin in patients with ovarian carcinoma relapsing after treatment with carboplatin. Eur J Cancer 30A:3033.
- Averill DA, Su C (1999) Sensitization to the cytotoxicity of adriamycin by verapamil and heat in multidrug-resistant Chinese hamster ovary cells. Radiat Res 151:694-702.
- Raaphorst GP, Doja S, Davis L, et al. (1996) A comparison of hyperthermia cisplatin sensitization in human ovarian carcinoma and glioma cell lines sensitive and resistant to cisplatin treatment. Cancer Chemother Pharmacol 37:574-580.
- Wallner KE, DeGregorio MW, Li GC (1986) Hyperthermic potentiation of cisplatinum(II) cytotoxicity in Chinese hamster ovary cells resistant to the drug. Cancer Res 46:6242-6245.
- Westermann AM, Grosen EA, Katschinski DM, et al. (2001)A pilot study of whole body hyperthermia and carboplatin in platinum-resistant ovarian cancer. Eur J Cancer 37:1111-1117.
- Wiedemann GJ, Robins HI, Gutsche S, et al. (1996) Ifosfamide, carboplatin and etoposide (ICE) combined with 41.8 degrees C whole body hyperthermia in patients with refractory sarcoma. Eur J Cancer 32A:888-892.
- Bull JM, Bigham CJ, Cronau LH, et al. (1992) A phase II study of whole body hyperthermia with mitomycin C (mito-C) and continuous iv (civ) 5-fluorouracil (5-FU) in colon cancer (meeting abstract). Proc Annu Meet Am Assoc Cancer Res 33:A1500.
- Bull JM, Cronau LH, Newman BM, et al. (1992) Chemotherapy resistant sarcoma treated with whole body hyperthermia combined with 1-3-bis(2-chloroethyl)-1-nitrosourea (BCNU). Int J Hyperthermia 8:297-304.
AuthorS
Friedrich Douwes • Juri Bogovic • Ortrun Douwes • Friedrich Migeod • Christoph Grote
Acknowledgments
The authors wish to thank Dr. Shari Lieberman for generous support in correction of the manuscript.
Download
You can download this article: Download PDF